Several gene and cell therapy treatments for rare and monogenic diseases and cancer are moving into late-phase clinical trials and commercial production.
These advances have led to more regulatory oversight on the manufacturing processes to produce viral vectors used for these treatments.
Regulatory expectations for gene therapy are discussed in the light of the latest FDA Guidance to Industry (Jan 2020).
The production cells used in the viral vector manufacturing process are considered critical raw materials and as part of the need to have a well-controlled process that provides consistent manufacture of the product, it is important to characterize the production cell line.
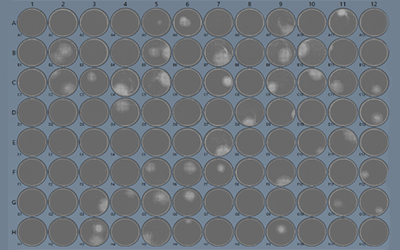
Single cell cloning of the production cells will allow for the selection of cells with increased vector productivity and provide a homogenous stable population of cells that can be used for the manufacturing process. Verification of clonality is of the utmost importance and in the presentation Seth demonstrates the use of the Solentim Cell Metric® to verify clonality of each cell and track its outgrowth. The Cell Metric has been successful in determining clonality and identifying the single cell outgrowth of mammalian and insect cell lines at Patheon Viral Vector Services within ThermoFisher.
In their workflow, single cell cloning is performed with single cell seeding, and the Cell Metric whole well imager is used on the day of seeding (d0) to verify where the single cells are in each well of a 96 well plate. The Cell Metric tracks the clone’s outgrowth and generates a Clonality Report, tracking the cell from seeding to time of passage. Clones can also be tracked during scale up via imaging various plate sizes before passage into larger flasks. The ability to document single cells and follow their growth provides the necessary support and assurance of clonality for inclusion in their clients’ IND submissions to the regulatory authorities.
Example Clonality Reports are presented by Seth for HEK293 and Sf9 producer cell lines
Please fill out the form to view this content.